Many businesses immediately raised a question in their minds, do I have to pass the US FDA inspection to sell cosmetics and skin care products?
Except for colorants, cosmetics and their ingredients do not need to be approved by the FDA before they are marketed, but the product ingredients and labeling must be in accordance with FDA regulations, and no fake or FDA prohibited substances must be added.
We know that there are many international brands selling cosmetics and skin care products in China. This year, China’s “Double Eleven” carnival shopping festival has set a record for e-commerce. However, compared with previous years, most Pakistani manufacturers’ sales this year It is not as expected. It may be an exaggeration to say that the fiasco, but the fierce international competition is an indisputable fact.
Facial Mask Market Example
For example, in addition to the double-sided attack from China and South Korea in the facial mask market, when competing with brands from all over the world, “Made in Pakistan” is like a small shrimp in the Pacific. When facing a dazzling array of international products, Pakistan. The sale of goods is also eaten away by whales.
But since foreign brands can go to China to fight with us, why don’t we turn our eyes to another broad market, the United States.
The American market and the Chinese market have different tonality. There is no need to cut prices arbitrarily, but to pay more attention to product quality and consumer experience. It is also a choice worth considering developing the American Amazon market, create new reputation and brand, and create new business opportunities. (For more FAQs about FDA Inspection Process visit Marketingskull.com)
FDA Regulations
Regardless of the source of the ingredients, the product must be safe Yu, to protect the rights and interests of consumers.
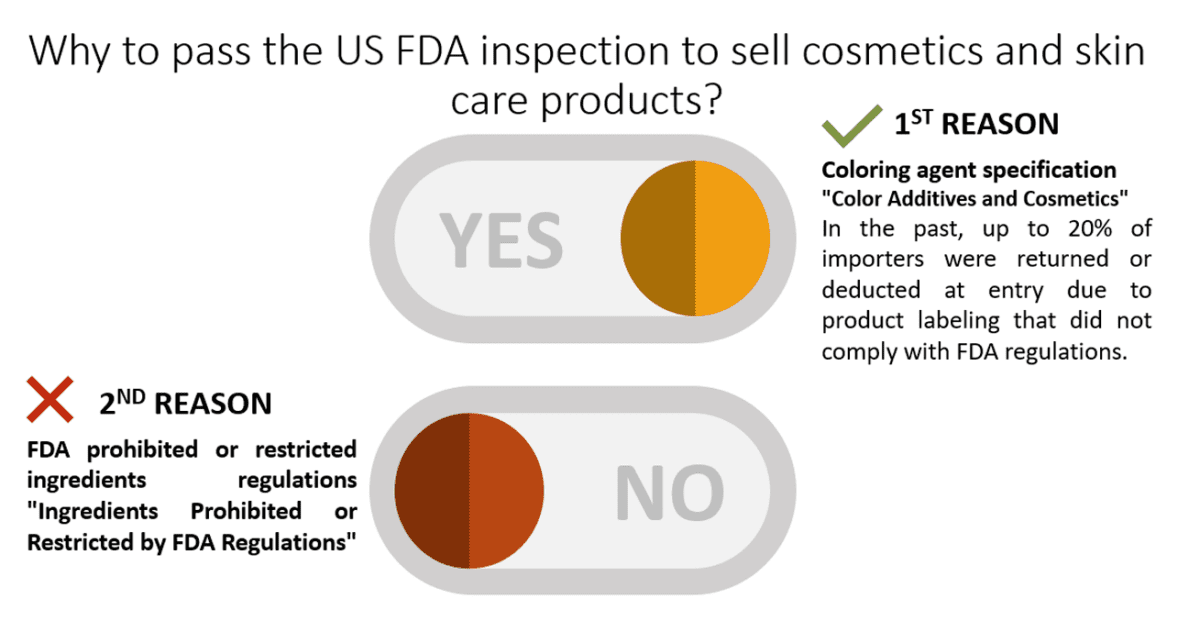
- FDA prohibited or restricted ingredients regulations “Ingredients Prohibited or Restrictedby FDA Regulations“
- Coloring agent specification “Color Additives and Cosmetics”
In the past, up to 20% of importers were returned or deducted at entry due to product labeling that did not comply with FDA regulations. Therefore, many assisting companies on the Internet have assisted businesses in making product labels that comply with FDA regulations and coordinated trademark design. If you have any needs in this regard, you can also ask Marketingskull.com for assistance.
Will it be troublesome if I want to sell cosmetics in the United States?
It is deceptive to say that it is not troublesome, but in fact it is not as difficult as you think.

I like reading a post that will make men and women think. Also, thanks for allowing me to comment!